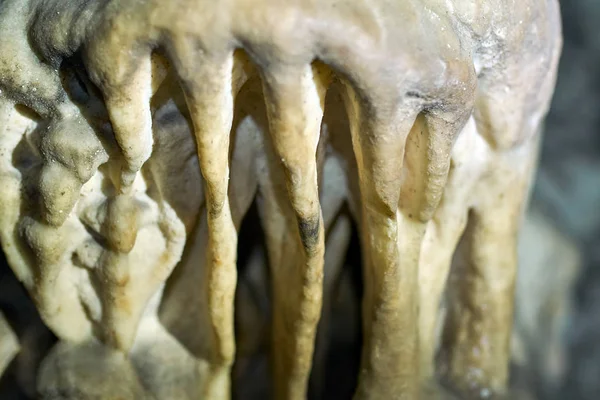
Speleothems are secondary mineral deposits formed in caves when mineral-rich waters precipitate solids onto ceilings, walls, or floors; the great majority are calcium carbonate features that decorate caves in karst landscapes. Typical expressions include stalactites, stalagmites, draperies, columns, flowstone, coralloids (“cave popcorn”), and cave pearls, whose morphologies reflect whether water drips, flows, seeps, condenses, or ponds. These deposits are distinct from erosional cave features and are central to both cave aesthetics and paleoenvironmental research, particularly because their layered growth can be accurately dated. Encyclopaedia Britannica;
U.S. National Park Service.
Etymology and definition
- –The term derives from Greek spelaion (cave) and théma (deposit), and in usage refers to any secondary mineral deposit in a cave, irrespective of specific mineralogy. In public interpretation and scientific literature alike, stalactites (ceiling-hanging) and stalagmites (floor-rising) are the best-known kinds.
National Park Service;
Encyclopaedia Britannica.
Geologic setting and formation
- –Most speleothems form in carbonate caves developed in Limestone within Karst terrains. Percolating meteoric water acquires CO₂ from soil and epikarst, producing carbonic acid that dissolves Calcite (CaCO₃); when the water emerges into an air-filled cavity, CO₂ degasses and calcite re-precipitates as a solid coating or dripstone. The cyclic dissolution–precipitation pathway reflects bedrock chemistry, CO₂ partial pressures, evaporation, and hydrology.
National Park Service;
Encyclopaedia Britannica. Kinetic studies show that calcite dissolution and subsequent re-precipitation rates depend on both surface-reaction kinetics and mass transport, providing a geochemical basis for cave speleothem growth under varying pH and CO₂ conditions.
USGS Publications Warehouse.
Mineralogy
- –The majority of speleothems are calcareous, composed of calcite or its polymorph aragonite, though sulfate minerals (e.g., gypsum, epsomite, mirabilite) occur especially in dry caves where evaporation dominates, and opaline silica and other phases are locally present. Mineralogy, impurity content, and crystallography influence translucence and color.
Encyclopaedia Britannica;
U.S. Geological Survey.
Morphological types
- –Stalactites originate from pendant droplets on ceilings, often beginning as hollow “soda straws” that later infill; stalagmites grow upward from drips that impact the floor; when they join, a column forms. Flowstone develops from films of flowing water; thin draperies (curtains) hang along fracture-controlled lines; helictites twist due to capillarity and microflow; coralloids (“cave popcorn”) encrust walls; rimstone dams build terraces at pool margins; cave pearls are concentric calcite spheres that accrete around a nucleus in agitated pools.
National Park Service;
Encyclopaedia Britannica;
U.S. Geological Survey.
Growth dynamics and rates
- –Growth rates vary across orders of magnitude with drip chemistry, cave-air pCO₂, temperature, and hydrology. U–Th dated examples show average rates on the order of a few tenths of a millimeter per year in many temperate caves, while other records document much slower growth—down to hundredths or less of a millimeter per year—over glacial–interglacial timescales; conversely, some sites achieve millimeter-per-year growth under high supersaturation and favorable ventilation. Case studies illustrate ~0.2–0.7 mm/yr in Alpine caves and ~0.3–0.4 mm/yr in the Little Carpathians, whereas Mediterranean records demonstrate intervals with rates below 0.02 mm/yr.
Journal of Seismology;
Climate of the Past (Copernicus); discussion and references therein include Boch et al. (2006, 2010). Software and monitoring studies further emphasize the sensitivity of growth to seasonal cave processes and supersaturation thresholds.
Geosciences (MDPI).
Dating methods
- –Uranium–thorium (U–Th) disequilibrium dating is the standard radiometric tool for calcite/aragonite speleothems, enabling high-precision chronologies typically up to ~600–650 thousand years under optimal conditions, dependent on uranium content and detrital corrections. Methodological advances in mass spectrometry have refined precision and sample-size requirements.
Elements. For older material or where U–Th is limited, uranium–lead (U–Pb) dating extends speleothem ages into the Middle Pleistocene and beyond, with recent in situ LA-ICP-MS approaches improving spatial resolution and expanding the dateable range; U–Pb has yielded ages exceeding one to two million years in appropriate flowstones.
Geochronology (Copernicus);
USF Karst Information Portal (Quaternary Geochronology).
Paleoclimate and paleoenvironmental proxies
- –Speleothems record climate through time-varying growth and geochemistry. Stable oxygen (δ¹⁸O) and carbon (δ¹³C) isotopes respond to moisture source, rainfall amount, temperature, cave ventilation, vegetation and soil-respired CO₂, water–rock interaction, and prior calcite precipitation; careful site-specific interpretation is required.
USGS Karst Interest Group 2024 Proceedings. The classic “Hendy test” evaluates equilibrium deposition along a growth lamina to assess whether isotopic variations are climatic rather than kinetic.
Geochimica et Cosmochimica Acta. Trace elements (e.g., Mg/Ca, Sr/Ca) and fabrics complement isotopes; annual to sub-annual laminae permit ring-width climatology analogous to tree rings.
NASA Earth Observatory. Global archives curated by NOAA’s World Data Service house thousands of speleothem records, including benchmark datasets for events such as the 8.2 ka cold event and monsoon variability.
NOAA NCEI dataset overview;
NOAA NCEI dataset overview.
Biological influences
- –Microorganisms can mediate carbonate precipitation by altering pH, alkalinity, or supplying nucleation sites, leading to microbially induced carbonate precipitation that may leave diagnostic microtextures and occasionally influence δ¹³C at the microscale, even if bulk records are dominated by abiotic processes such as CO₂ degassing.
Biogeosciences. Cultured cave isolates demonstrate active calcite/aragonite precipitation under low-temperature cave-like conditions, underscoring potential contributions of cave microbiota to speleothem initiation and growth.
PMC (Extremophiles study).
Distribution and examples
- –Speleothems occur on all continents in caves from humid tropics to periglacial settings, with mineralogy and morphology adapting to cave climate: gypsum flowers and snowballs favor drier caves, while calcite dripstone dominates in well-ventilated systems supplied by carbonate-saturated drips. U.S. examples such as Carlsbad Caverns and Mammoth Cave exhibit extensive speleothem assemblages, including stalactites, stalagmites, draperies, and coralloids.
U.S. Geological Survey;
National Park Service.
Conservation and management
- –Speleothems are fragile, grow slowly relative to human timescales, and are easily damaged by touch, breakage, dust, or altered cave ventilation; conservation practice emphasizes avoiding contact, controlling visitor access, and managing lighting and airflow.
National Park Service.
Selected types at a glance
- –Stalactite: ceiling pendant, commonly evolving from soda straws.
National Park Service.
- –Stalagmite: drip-impact mound on floors; may merge with stalactites to form columns.
National Park Service.
- –Drapery/curtain: thin calcite sheets along ceiling fractures.
Encyclopaedia Britannica.
- –Flowstone: sheet-like calcite from films of flowing water.
National Park Service.
- –Coralloid (“cave popcorn”): knobby encrustations on walls and speleothem surfaces.
U.S. Geological Survey.
- –Cave pearl: concentric calcite spheres formed in agitated pools.
Encyclopaedia Britannica.
Internal links: Karst, Limestone, Calcite, Stalactite, Stalagmite, Paleoclimatology.